For the treatment of hypertension in combination with other antihypertensive drugs, to lower blood pressure in adult patients who are not adequately controlled on other drugs.


Help lower difficult-to-control hypertension by adding once-daily TRYVIO.1
TRYVIO—the first and only dual endothelin receptor antagonist for systemic hypertension—takes a different path to lower blood pressure.1,2
Efficacy
TRYVIO significantly reduced systolic blood pressure by targeting the endothelin pathway1
In patients taking TRYVIO and at least 3 blood pressure medications (n=243), TRYVIO demonstrated statistically superior blood pressure reductions vs placebo1
Primary endpoint: change in sitting SBP (SiSBP) from baseline to week 41
Week 4 sitting trough SBP

- Reduction in sitting trough SBP for the placebo with antihypertensive background therapy group (n=244) was 11.6 mm Hg for a difference of 3.8 vs TRYVIO (97.5% CL, [-6.8, -0.8]; P=0.0043).1,b
aCalculated as least squares mean.1
bStatistically significant at the 2.5% level as prespecified in the testing strategy.1
Blood pressure reduction appeared consistent among subgroups defined by1:
- Age
- Sex
- Race
- BMI
- Baseline eGFR
- Baseline UACR
- Medical history of diabetes
PRECISION: Study design
A robust trial of patients taking standardized antihypertensive treatment1
The safety and efficacy of TRYVIO were evaluated in a 3-part, phase 3 multicenter study of adults (N=730) with SBP ≥140 mm Hg who were prescribed 3 or more antihypertensive medications.
- Part 1 was 4 weeks long, double-blind, randomized, and placebo-controlled. Patients received TRYVIO 12.5 mg, aprocitentan 25 mg, or placebo in a 1:1:1 ratio.
- Part 2 was 32 weeks long and single (patient)–blinded. All patients received aprocitentan 25 mg.
- Part 3 was a 12 weeks long, double-blind, randomized, and placebo-controlled withdrawal period. Patients were re-randomized to aprocitentan 25 mg or placebo in a 1:1 ratio before part 3 began.
Primary/secondary endpoints
The primary and key secondary endpoints were changes in unattended automated office systolic blood pressure from baseline to week 4 and from week 36 to week 40, respectively.
Select inclusion criteria1,2:
- Unattended sitting office SBP ≥140 mm Hg
- History of uncontrolled blood pressure despite ≥3 antihypertensive medications for at least 1 year
Select exclusion criteria3:
- Confirmed severe hypertension (grade 3)
- Major cardiovascular, renal, or cerebrovascular medical complications in the past 6 months or NYHA stage III-IV heart failure
- NT-proBNP levels ≥500 pg/mL
- eGFR <15 mL/min/1.73 m2
At baseline, 63% of patients reported taking ≥4 antihypertensive drugs1
ARB = angiotensin II receptor blocker; BMI = body mass index; CCB = calcium channel blocker; CL = confidence limits; eGFR = estimated glomerular filtration rate; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; SBP = systolic blood pressure; UACR = urine albumin-creatinine ratio.
Safety
Demonstrated long-term safety profile (48 weeks)1
The safety profile of TRYVIO was evaluated during a 48-week study of 724 patients who had uncontrolled blood pressure (SBP ≥140 mm Hg) despite taking 3 or more antihypertensive medications.1
The most frequently reported adverse reactions to TRYVIO during the 4-week, double-blind phase of the study were edema/fluid retention and anemia.1
Adverse Reactions1,c
| ADVERSE REACTION |
|---|
| Edema/fluid retention |
| Anemia |
| TRYVIO 12.5 mg n=243 |
|---|
| 9.1% |
| 3.7% |
| PLACEBO n=242 |
|---|
| 2.1% |
| 0 |
cReported with a frequency of ≥2% in TRYVIO-treated patients and greater (≥1%) than in placebo-treated patients during the initial 4-week, double-blind, placebo-controlled phase.
How it works
Takes a different path to help your patients lower their blood pressure
TRYVIO is the first and only dual ERA for the treatment of systemic hypertension.1,2
TRYVIO mechanism of action (MOA)
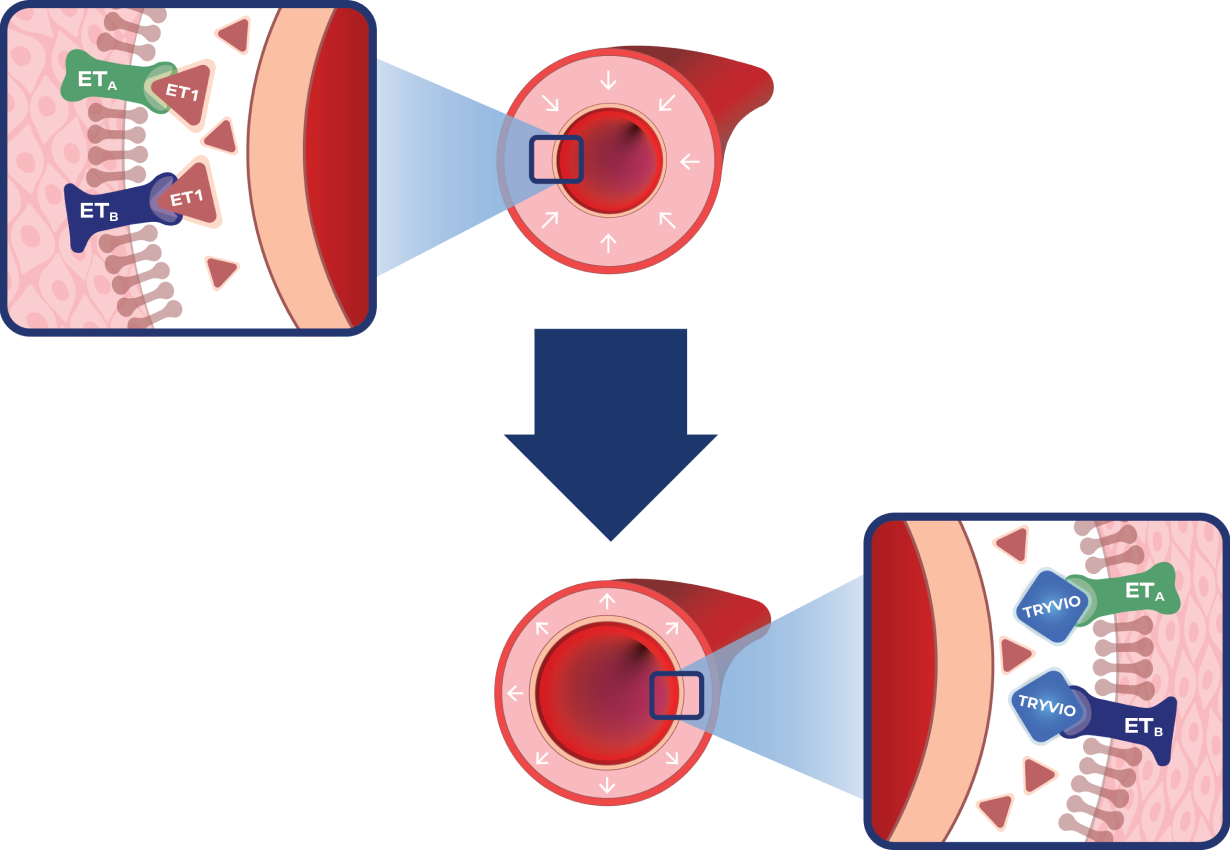
Hypertensive State
Overactivation of the endothelin system induces vasoconstriction and remodeling via binding of ET-1 to ETA and ETB receptors.1,4,5
The TRYVIO Effect
TRYVIO inhibits the endothelin system by blocking ETA and ETB receptors.1
The endothelin system is one of several key pathways in hypertension and has not been successfully targeted by existing systemic hypertension therapies.2,6
DOSING
Take the pressure down with once-daily dosing1
- TRYVIO may be taken with or without food.
- Swallow tablets whole.
- If a dose is missed, skip the missed dose and take the next dose at the regular time. Do not take two doses on the same day.
- Exclude pregnancy before initiating treatment with TRYVIO in females of reproductive potential.
No dose adjustment is required in patients with mild to severe renal impairment (eGFR ≥15 mL/min)1
- TRYVIO is not recommended in patients with kidney failure (eGFR <15 mL/min) or on dialysis.
- Patients with renal impairment are at increased risk of edema and fluid retention.
In clinical studies1,2,7,8:
- No clinically relevant drug-drug interactions were reported.
- No evidence of increased incidence of hyperkalemia was reported.
Prescribing
Savings and support to help start and stay on TRYVIO
TRYVIO is available through Walgreens Specialty Pharmacy
Walgreens Specialty Pharmacy will automatically apply the appropriate savings program and determine coverage and out-of-pocket cost for your patients.
To get started, simply e-prescribe your patients’ prescriptions with refills* in your EHR system to the Walgreens Specialty Pharmacy below:
Walgreens Specialty Pharmacy
NCPDP: 3974157
NPI# 1972560688
130 Enterprise Dr. | Pittsburgh, PA 15275
Toll Free Phone Number: 888-347-3416
Fax Number: 877-231-8302
*Refills are not required for the patient to participate in the free trial offer; however, if the Rx is written with refills, Walgreens can determine insurance coverage while the free trial is initiated to help prevent future delays. Walgreens Specialty Pharmacy may request additional prescriptions if needed.
Two ways patients can save:
Free Trial Offer

TRYVIO Savings

aLimitations apply. This offer is good for a 30-day (maximum 30 tablets; one time use) free trial of TRYVIO at no cost to your patient. See full Terms and Conditions.
bLimitations apply. For commercially eligible patients only. This offer is not valid under Medicare, Medicaid, or any other federal or state program. Patient must be 18 years of age or older and a resident of the United States. See full Terms and Conditions.
EHR = electronic healthcare record.
Stay up to date on TRYVIO news